Which of the Following Has Eight Valence Electrons
Hence option E is correct. The atoms will be In general elements that have four to eight electrons in their valence shell are Which of the following statements isare CORRECT.

Physical Chemistry Can An Atom Have More Than 8 Valence Electrons If Not Why Is 8 The Limit Chemistry Stack Exchange
Helium atoms have 2 valence electrons while atoms of the other elements in the group all have 8 valence electrons.
. Chlorine is a halogen gas located in group 17 p block Chlorine has an atomic number of 17 and an atomic mass of 35 Electron configuration. Valence electrons in Aluminum Al 3. Molecules or ions in.
From the electronic configuration of the oxygen we can see that there are six valence electrons present in its valence shell. A Ca B Rb C Xe D Br- E All of the above have eight valence electrons. That depends on the element in question.
B the energy given off when gaseous ions combine to form one mole of an ionic solid. Chemical reactions result in the gain loss or rearrangement of valence electrons. The 16 electrons are arranged in the configuration 286.
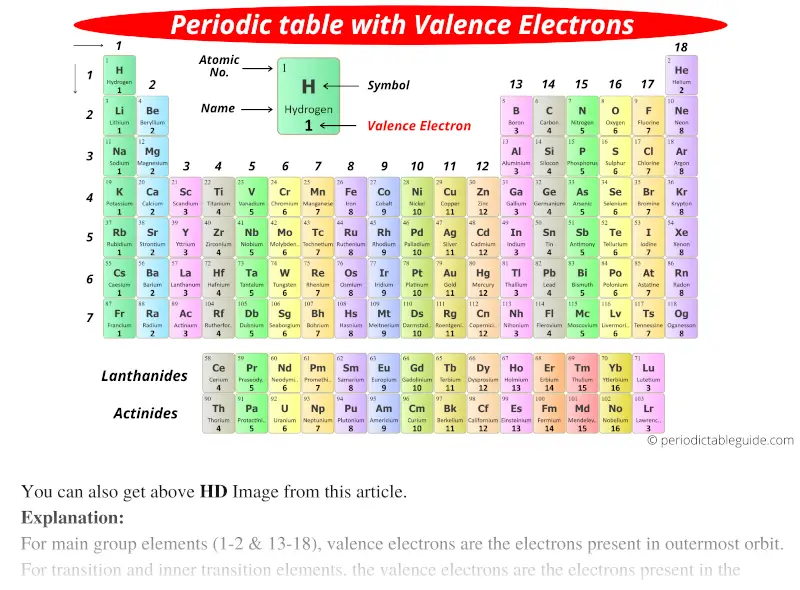
How is helium different from the other elements in this group. How many grams of cog are there in 745 ml of the gas at 0933 atm and 30o c. Electrons in the outermost shell is known as valence electrons.
For main group elements the number of valence electrons equals eight minus the elements group number. Copper is a good conductor of electric current because it has eight or more electrons in its valence band. Additional precipitate is forming.
Of the 16 electrons 6 are valence while 10 are core electrons making it the correct answer. A the energy required to convert a mole of ionic solid into its constituent ions in the gas phase. Na has 8 valence electron in its valence shell.
Molecules or ions with an odd number of electrons 2. 19ac to definition of lattice energy it is nothing b View the full answer. Valence electrons in Silicon Si 4.
Cl - has 8 valence electrons in its valence shell. The ions are now combining to. 1 question Which of the following has eight valence electrons.
Mercury turns to vapor at 62988 k how much heat is lost 175 g of mercury vapor at. Valence electrons in Fluorine F 7. Answer the questions below for an element that has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s1.
Too much solid has dissolved. The solution is unsaturated. Using a value of ksp 18 x 10-2 for the reaction pbcl2 pb2aq 2cl -aq.
Atoms can have anywhere from 1 to 8 valence electrons. Asked Jun 29 2017 in Chemistry by Pique. Valence electrons in Phosphorus P 5.
Neon krypton argon and radon. Which of the following does not have 8 valence electrons. Valence electrons in Sodium Na 1.
A stable arrangement of eight valence electrons. Sulfur has a total of 16 electrons. C X e The atomic number of Xenon is 54 The electronic configuration of Xe is K r 4 d 10 5 s 2 5 p 6 It has 8 electrons in its valence shell and thus is a.
1 Which of the following has eight valence electrons. Helium because it have two electrons in outermost shell. Which of the following has eight valence electrons.
If the value of ksp was determined to be only 12 x 10-2. Ti4 has 8 valence electron in its valence shell. Which of the following has 8 valence electrons Answers.
All of the noble gases except helium have 8 valence electrons. A Ti4 B Kr C Cl- D Na E all of the above 2 Which of the following does not have eight valence electrons. For the main group elements Groups 1213-18 the.
Valence electrons in Magnesium Mg 2. Lattice energy is ________. Answer is option c.
Kr has 8 valence electron in its valence shell. Another question on Chemistry. Valence electrons in Oxygen O 6.
E All of the above have eight valence electrons. Molecules or ions in which an atom has fewer than 8 valence electrons 3. Valence electrons in Sulfur S 6.
Advertisement Advertisement allrounderguruji2004 allrounderguruji2004 Answer. Valence electrons in Neon Ne 8. In an oxygen element each oxygen atom contains eight electrons in its valence shell.
A r 3 d 10 4 s 2 4 p 6 Since R b has 8 electrons in its valence shell and resembles the configuration of a noble gas Krypton it has a complete octet. AAr bNe cKr dHe 2 See answers Advertisement Advertisement ajayaggarwal1282 ajayaggarwal1282 Answer. Helium is in group 18 of the periodic table.
18 except Sr remaining four Ti4 Xe RbCl- are having 8 valence electrons in outermost shell but in case Sr it has electronic configuration of Kr4s1 and it only 1valence electron. Core electrons are not involved in bonding or in chemical reactions. However the chemical element Hydrogen is an exception to the octet rule because it is only able to hold a maximum of two 2 valence electrons in its outermost shell to become full.
Sign up to view the full answer. Therefore atoms of chemical elements bond in order to attain the electronic configuration of a noble gas ie a full valence shell which comprises of eight 8 electrons. Oxygen has atomic number of 8.
How many valence electrons can an atom have. Cl-Na Ti4 Kr all of the above. Rubidium has an atomic number of 37 The electronic configuration of R b will be.

Periodic Table With Valence Electrons Labeled 7 Hd Images
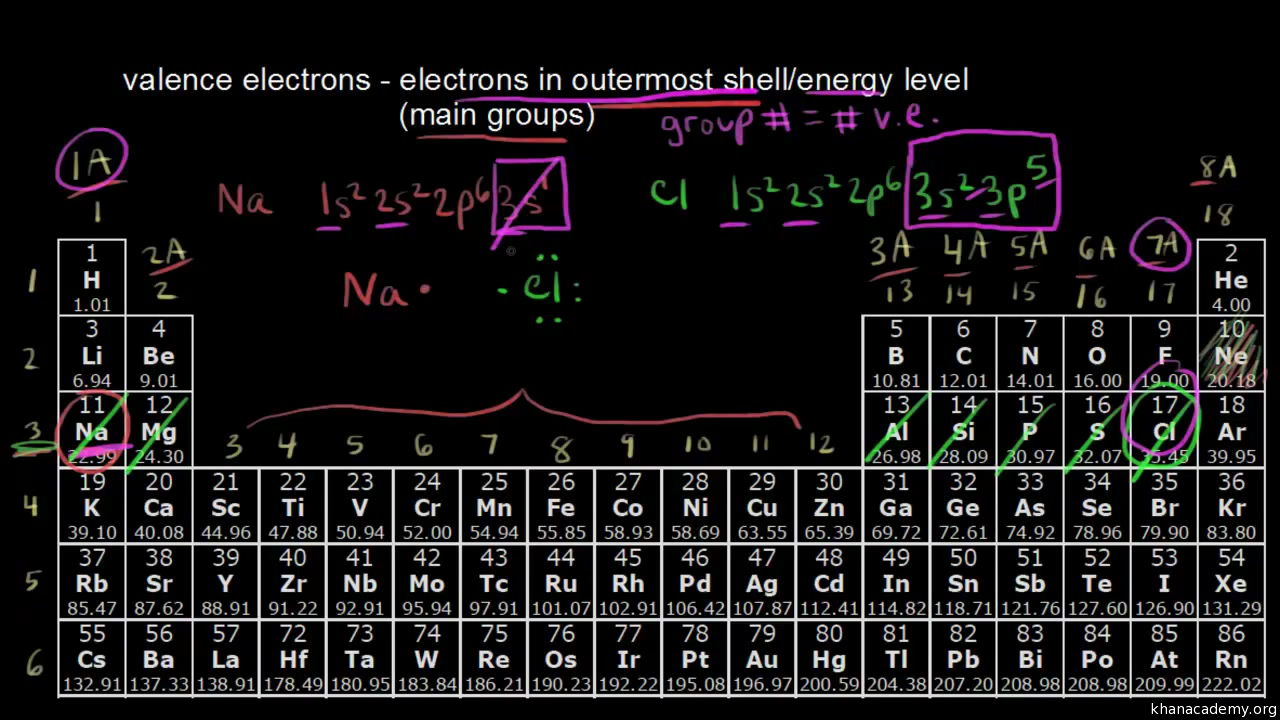
Counting Valence Electrons For Main Group Elements Video Khan Academy

Valence Electrons Characteristics And Determination Of Valence Electrons

How Many Valence Electrons Does Krypton Kr Have

Finding The Number Of Valence Electrons For An Element Youtube

Lewis Diagram Of Xenon Difluoride Worked Example Video Khan Academy

10 6 Valence Electrons Chemistry Libretexts

What Are Valence Electrons Chemtalk
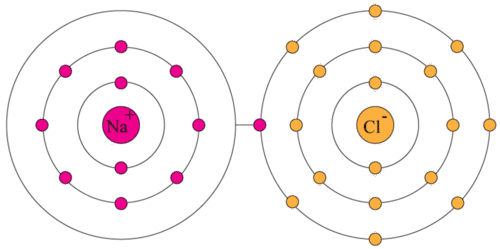
Valence Electrons Read Chemistry Ck 12 Foundation

How Many Valence Electrons Each Element Has

Graphical Abstract The Last Valence Electrons Left On An Ion Are Download Scientific Diagram

Valence Electrons Energy Levels Of Elements How Many Electrons Does Each Element Have Video Lesson Transcript Study Com

How Many Valence Electrons Does Argon Ar Have

How Many Valence Electrons Does Sulfur S Have



Comments
Post a Comment